Find out the total number of valence electrons. Remember Phosphorous is below Period 2 on teh Periodic Table and can hold more than 8 valence.
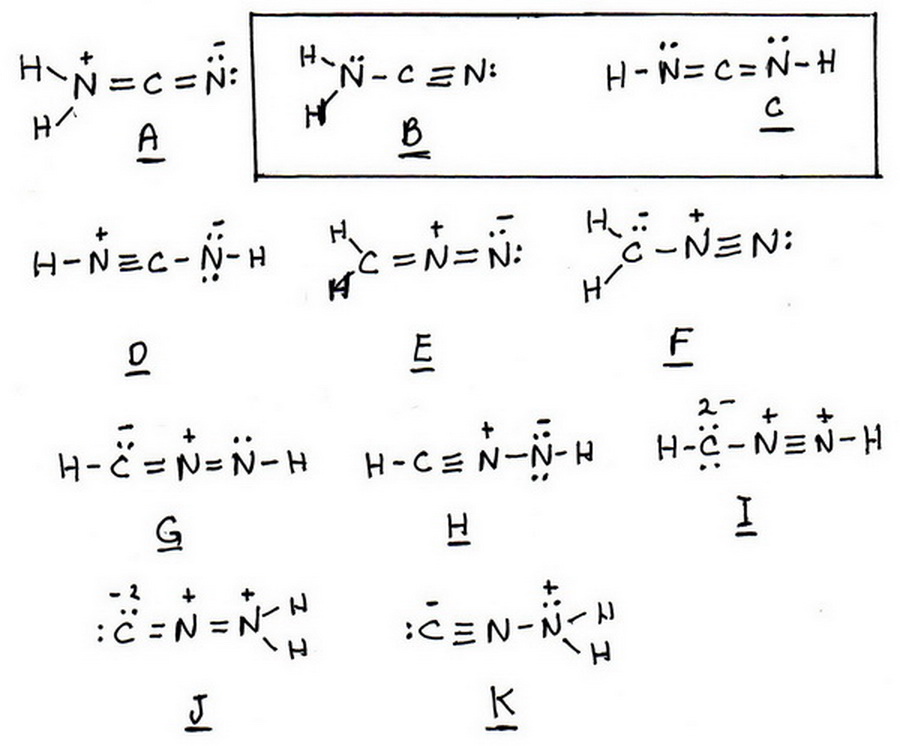
What Isomeric Lewis Structure Of Cn 2h 2 Has No Formally Charged Atoms Socratic
They are also called dot electron diagrams of molecules.

. Draw the Lewis structures for the following four molecules Structures without work shown will be marked incorrect. So total 213 24. Drawing the Lewis Structure for Cl 3 PO.
A step-by-step explanation of how to draw the CH2N2 Lewis Dot Structure DiazomethaneFor the CH2N2 structure use the periodic table to find the total numbe. Answer 20 Watch For unlimited access to Homework Help a Homework subscription is required. Minimize formal charges and include lone pairs.
BCl3 Lewis Structure. 2 Answers The structure of NCNH₂ is shown as follows. From your diagram around each oxygen atom there are 2 lone pairs thats 4 electrons and it is conceived to get a half share of the four electrons in the O I bond.
A step-by-step explanation of how to draw the BCl3 Lewis Dot Structure Boron trichlorideFor the BCl3 structure use the periodic table to find the total nu. Valence electrons of carbon atom 4. Minimize the formal charges and include all lone pairs.
Drawing of CH NH22 lewis structure step-by-step 1. You might think youve got the correct Lewis structure for Cl 3 PO at first. Google it you speng Maybe you like Help with Change in Electric Potential Ranking Task.
Alkanes are simplest organic compounds that consist of single bonded carbon and hydrogen atoms with the general formula CnH2n2. H 1 valence electron I 7 valence electrons O 6 valence electrons And so we gots 1 7 4 6 32 valence electrons to distribute. I am looking for some conceptual help with this physics problem.
Drawing the Lewis Structure for NH 2-. Head Of Garlic. Amazon Luna special offer for Prime members.
NH 2-isnt usually found by itself. Try Amazon Luna Now. Alkanes are solid liquid or gas at room temperature depending on the size of their moleculesTo learn detailed structures formulas and Physical Properties of Alkanes with FAQS and Videos Visit BYJUS for more information.
There are roughly 10 to 12 cloves to a head. The Lewis structure for NH 2-you have a total of 8 valence electrons. Minimize formal charges and include lone pairs.
Draw the Lewis structure with the lowest formal charges for ClF2. Here the ion is made up of three different atoms which are Carbon C Hydrogen H and Nitrogen N so first we have to figure out the valence electrons of these atoms separately. The system was introduced by sir GN Lewis in 1916 in his article titled Atom and Molecule.
Already have an account. Include nonzero formal charges and lone pair electrons in the. Be sure to put brackets along with a negative sign around the NH 2-Lewis structure when you are done to show that it is an ion with a negative charge.
However its quite common to find it in organic compounds. See the answer See the answer done loading. First of all we need to calculate the total valence electrons of this molecule B 3.
By soetrust March 16 2022 Leave a reply 1. Bryllant Baluyut Lv10 28 Jan 2021 Unlock all answers Get 1 free homework help answer. This Site Might Help You.
Draw the structure of NCNH2 where the atoms are connected as shown in the formula. Also one of these molecules has resonance structures for this compound make sure to include all resonance structures indicate formal charges for. Let us apply the lewis dot rules and try to draw the structure of boron trichloride.
Minced garlic roughly equals one clove. Draw the structure of NCNH2 where the atoms are connected as shown in the formula. Now boron is less electronegative which makes it the central atom.
Draw the structure of NCNH2 the atoms are connected as shown in the formula. Amazon Luna launches with freebies for Prime subscribers. Up to 256 cash back Draw the structure of NCNH 2 where the atoms are connected as shown in the formula.
The Lewis structure for Cl 3 PO requires you to place Phosphorous P in the center of the structure since it is the most electronegative. Solution for Draw the structure of NCNH where the atoms are connected as shown in the formula. Lewis Structure of Polyatomic Molecules.
Minimize formal charges and include lone pairs. For the best answers search on this site shorturlimavIKF. So youll need about 5 to 6 tsp.
A lewis structure structure is a representation of a covalent compound in which dots are used to show valence electrons lone pairs and bonding electrons. If you use more or less thats ok. Garlic is almost always to taste.
Minimize formal charges and include lone pairs.

Oneclass Draw The Structure Of Ncnh2 Where The Atoms Are Connected As Shown In The Formula Minimiz
File Cyanamide Svg Wikimedia Commons

Chemistry Net Dot Structure How Do I Draw A Lewis Structure For A Molecule Lewis Dot Structure Of Cyanamide

Chemistry Net Dot Structure How Do I Draw A Lewis Structure For A Molecule Lewis Dot Structure Of Cyanamide

Ch2n2 Lewis Structure How To Draw The Lewis Structure For Ch2n2 Youtube

Oneclass Draw The Structure Of Ncnh2 Where The Atoms Are Connected As Shown In The Formula Minimiz

0 comments
Post a Comment